
Uma introdução à coloração de rotina e especial


An Introduction to Routine and Special Staining
Routine H&E staining and special stains play a critical role in tissue-based diagnosis or research. By colouring otherwise transparent tissue sections, these stains allow highly trained pathologists and researchers to view, under a microscope, tissue morphology (structure) or to look for the presence or prevalence of particular cell types, structures or even microorganisms such as bacteria.
In the histopathology laboratory, the term “routine staining” refers to the hematoxylin and eosin stain (H&E) that is used “routinely” with all tissue specimens to reveal the underlying tissue structures and conditions. The term “special stains” has long been used to refer to a large number of alternative staining techniques that are used when the H&E does not provide all the information the pathologist or researcher needs.
Preparing Tissue for Staining
Before tissue can be stained and viewed, it must be prepared so that a very thin section, only one cell thick, can be cut and placed onto a microscope slide. This involves fixing the tissue (so it does not decay) then hardening and supporting it so that it can be cut to the very thin sections needed (typically 2–7 µm). There are two main techniques used for this, referred to as frozen sections and paraffin-embedded sections.
Frozen sections are used when answers are needed fast, typically during surgery where the surgeon needs to know the excision margin when removing a tumour. They are quick to produce, but typically do not create the same section quality of as the paraffin technique.. The process for frozen section preparation is as follows:
- Tissue is quickly frozen to preserve and harden it.
- The frozen tissue is sectioned in cryostat (a sectioning microtome in a freezing chamber) and placed on a microscope slide for staining.
- The section is fixed immediately before it begins to decay and is then stained.
When paraffin sections are to be prepared the specimen is first preserved with a fixative and then the tissue structure is supported by infiltrating the specimen with paraffin wax. The process is more time-consuming than creating frozen sections, but provides better quality staining in most cases and the resultant samples (referred to as blocks) can be stored almost indefinitely. The paraffin section process is as follows:
- Fixation preserves the tissue (typically using a formaldehyde- based solution).
- Grossing isolates the particular area of tissue to be sectioned.
- Tissue processing uses a sequence of reagents to replace an aqueous (water-based) environment with a hydrophobic one enabling tissue elements to be infiltrated with paraffin wax.
- Embedding allows specimen orientation and secures the specimen in a block of wax for section cutting and storage.
- Sectioning is done on a microtome that cuts very fine sections which are floated-out on a water bath then picked up and placed on microscope slides.
- The slides are then dried in an oven or on a hot plate to remove moisture and help the tissue adhere to the slide.
- The tissue on the slide is now ready for staining.
- The first staining step is de-waxing which uses a solvent to remove the wax from the slide prior to staining. This is always done as part of the staining process. When a stain is complete the section is covered with a coverglass that makes the preparation permanent.

Why H&E Staining is Routine
Hematoxylin and Eosin (H&E) staining is used routinely in histopathology laboratories as it provides the pathologist/researcher a very detailed view of the tissue. It achieves this by clearly staining cell structures including the cytoplasm, nucleus, and organelles and extra-cellular components. This information is often sufficient to allow a disease diagnosis based on the organization (or disorganization) of the cells and also shows any abnormalities or particular indicators in the actual cells (such as nuclear changes typically seen in cancer). Even when advanced staining methods are used, the H&E stain still forms a critical part of the diagnostic picture as it displays the underlying tissue morphology which allows the pathologist/researcher to correctly interpret the advanced stain.
In a clinical histology laboratory, all specimens are initially stained with H&E and special or advanced stains are only ordered if additional information is needed to provide a more detailed analysis, for example to differentiate between two morphologically similar cancer types.
Because of the volume of H&E staining needed, most clinical laboratories use fully automated systems and manual staining is now rare.
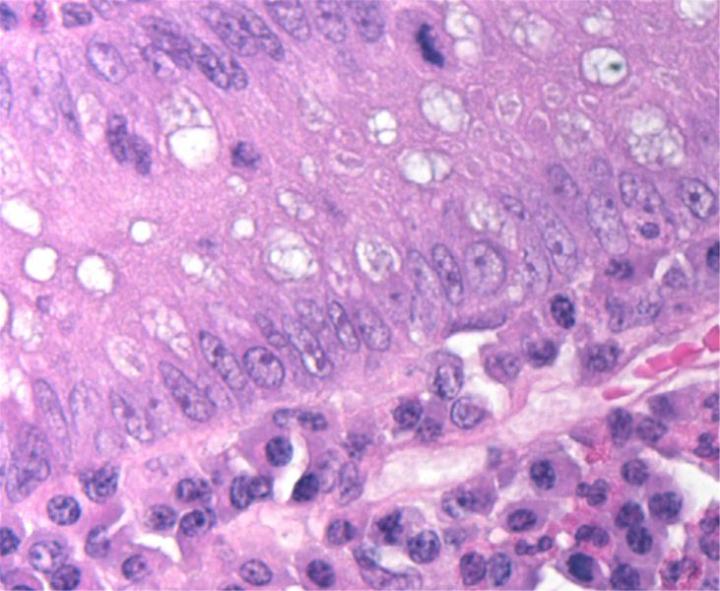

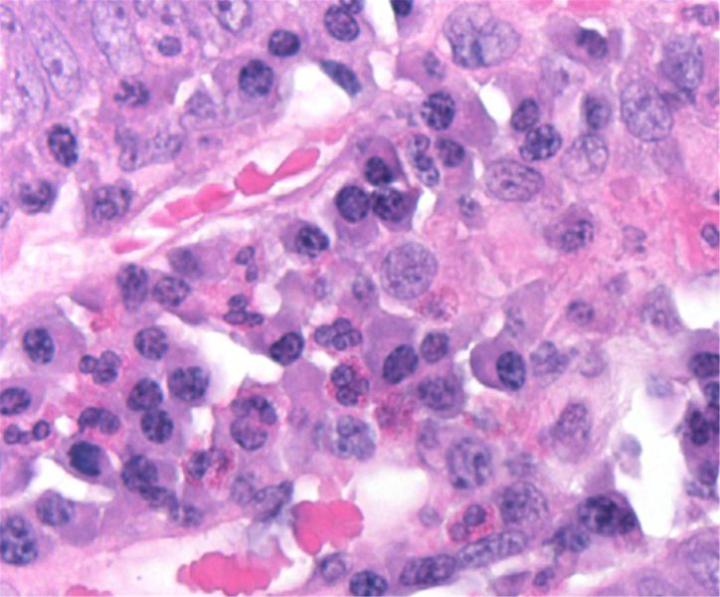

H&E Chemistry
The H&E stain uses two dyes: hematoxylin and eosin. This combination is used as the dyes stain different tissue elements.
Hematoxylin reacts like a basic dye with a purplish blue colour. It stains acidic, or basophilic, structure including the cell nucleus (which contains DNA and nucleoprotein) and organelles that contain RNA such as ribosomes and the rough endoplasmic reticulum.
Eosin is an acidic dye that is typically reddish or pink. It stains basic, or acidophilic, structures which includes the cytoplasm, cell walls, and extracellular fibres.
Dye origins
Hematoxylin is extracted from the logwood tree and purified. It is then oxidized and combined with a mordant (typically aluminium) to allow it to bind to the cell structures. Of the many hematoxylin preparations used in histology Gill’s hematoxylin, Harris's hematoxylin and Mayer's hematoxylin are the most popular.
Eosin is formed by a reaction between bromine and fluorescein. There are two eosin variants typically used in histology: eosin Y which is slightly yellowish and eosin B which is slightly bluish. Eosin Y is most popular.


Special Stains
The term special stains traditionally referred to any staining other than an H&E. It covers a wide variety of methods that may be used to visualize particular tissue structures, elements, or even microorganisms not identified by H&E staining.
Other methods of staining use immunohistochemistry or in situ hybridization to target specific proteins or DNA/RNA sequences. These methods were sometimes also included as members of the “special stains” family. However they are quite different in method and purpose and are now typically separated into a third category know as “advanced stains”.
While there are literally hundreds of special stains for all manner of purposes, only a few are used with any regularity in clinical histology. The variety of stains also means that special staining is not as automated as H&E staining. While many larger laboratories do use automated instruments for the more common stains, they still have an area for hand staining. The complexity of some stains also works against the uses of automation.
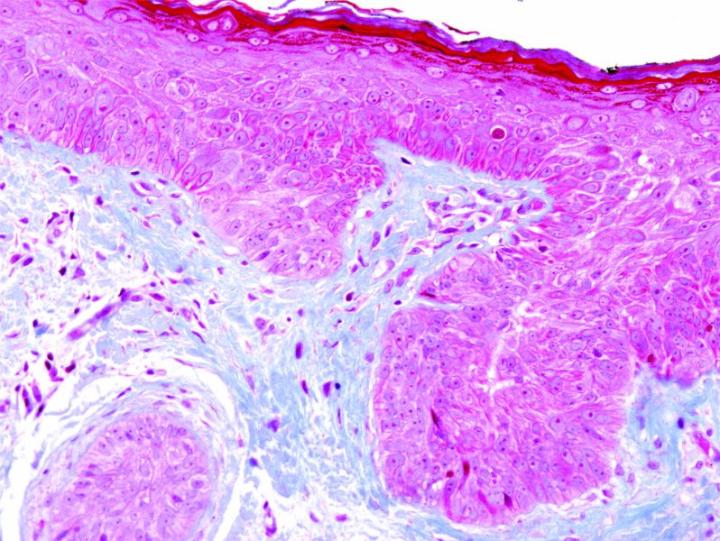
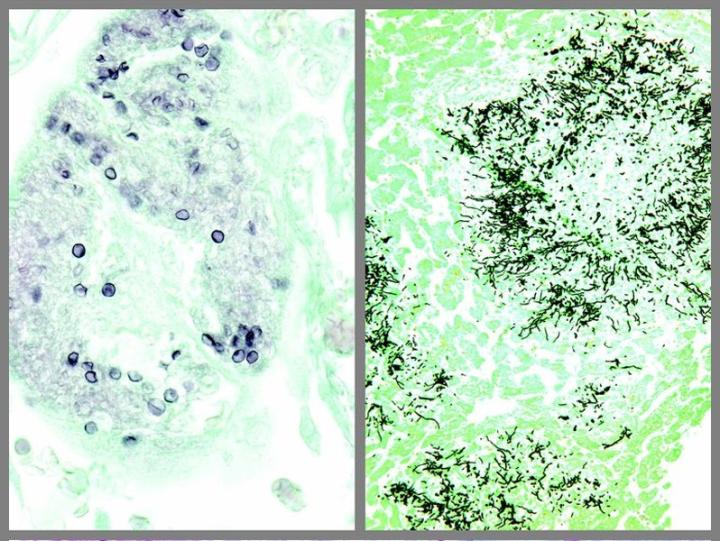
Some Common Special Stains
The images below illustrate some of the common special stains and their applications.
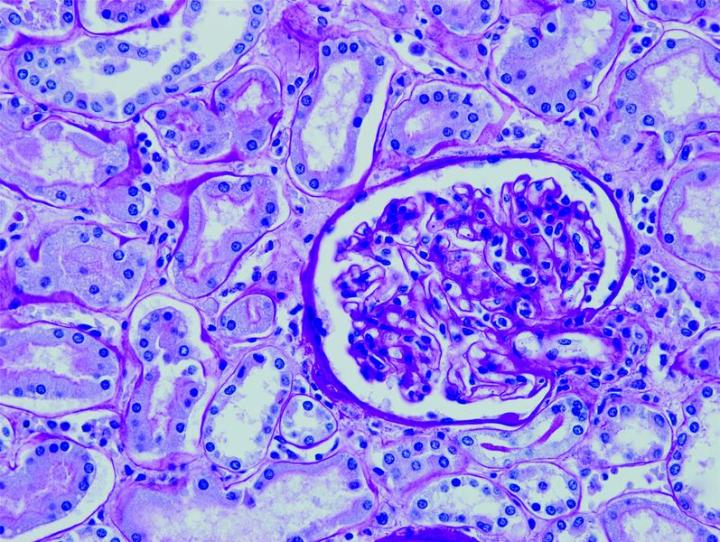


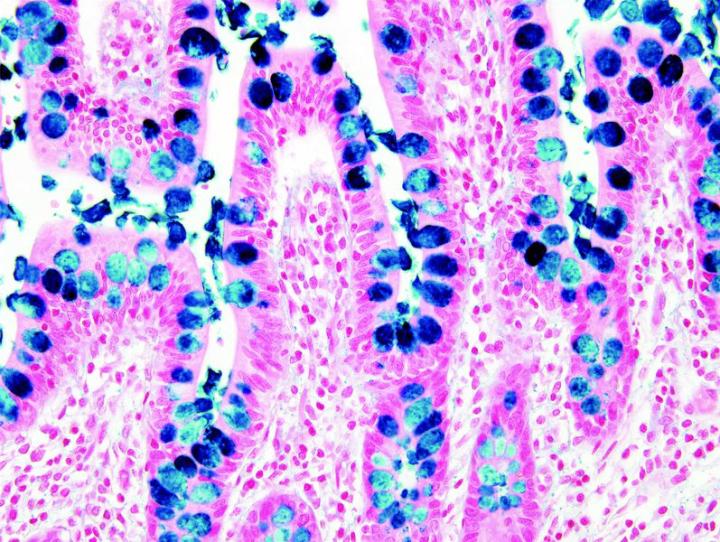
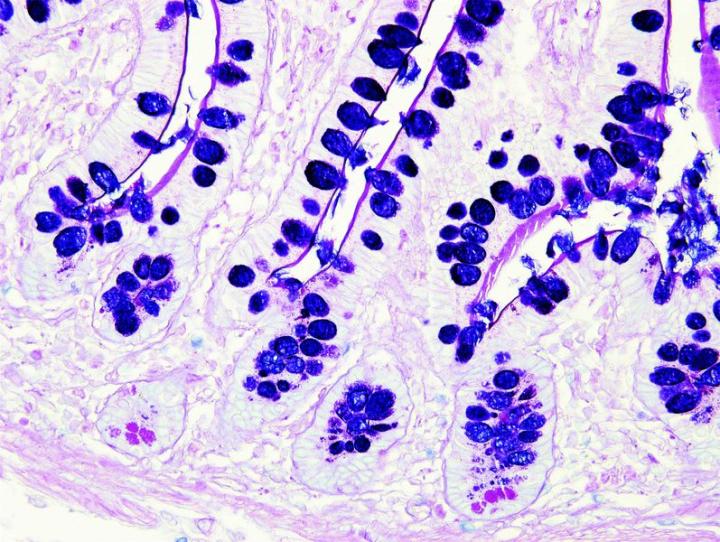
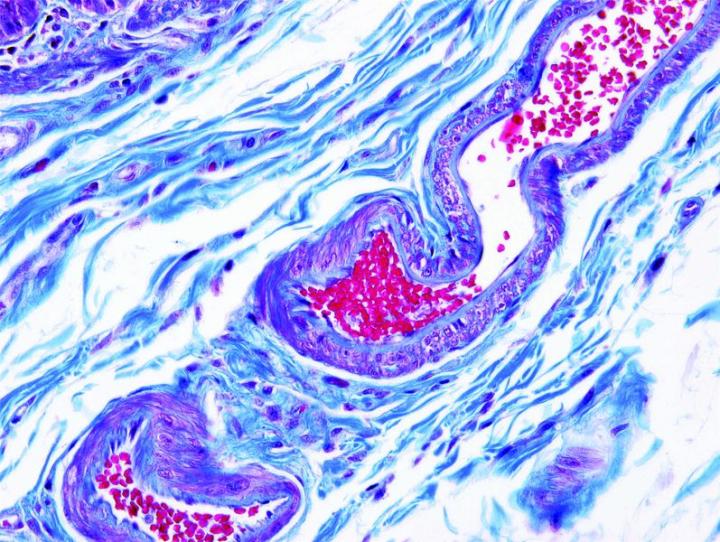

Steps to Better Special Stains
At Leica Biosystems, our vision is to advance cancer diagnostics and improve lives. One way we can achieve this vision is by helping improve staining quality. As we recognize that IHC and ISH quality doesn‘t begin at the stainer, this series looks at many different aspects of staining quality, and considers how future tests will influence improved diagnosis.
Understand the Stain
Know what you are trying to demonstrate with the stain you are performing.
Just “following the method” and not really knowing what should be seen in the finished section will lead to poor results.

Use a Positive Control
Always use a control slide known to contain the structure/ substance you are trying to demonstrate.
“If the structure/substance we are staining for is not visible in a slide, we assume it is not present.”

Use Accurate Timing
Use accurate timing.
Timing is always approximate. Inaccurate timing produces inconsistent results.

Consider Reagent Stability
Be aware of the shelf life of the reagents you are using. Some reagents or dye solutions deteriorate slowly while others are very unstable and must be made up fresh and used immediately. Others have to be left for some time to oxidize (ripen) before they can be used at all.
We assume all reagents can be used for an indefinite period.

Store Reagents Correctly
Store reagents correctly. Some require refrigeration because they are inclined to support the growth of fungi or molds. Others are light sensitive and require storage in the dark.
“All our reagents are stored on the shelf above the staining bench. Sometimes we see stray organisms in our sections.”

Adhere to the Method
Follow the protocol exactly.
Staff members achieve different results when supposedly using the same protocol.

Record Any Changes
Document any departure from the method you are using.
Sometimes when results are poor, it is difficult or impossible to work out why because protocol changes have not been recorded.

Standardize Washing Steps
Take particular care with washing steps. Standardize them as far as possible as they are frequently the cause of variable results.
Lab staff members use different washing techniques – some use vigorous agitation, others are much more gentle.

Set Up Microscope Carefully
Use microscopic control at crucial stages such as differentiation steps. Be aware of the effect of the microscope setup on the appearance of un-coverslipped (wet) sections; it can produce the appearance of false background staining.
For all methods, the level of staining is assessed by looking at the slide with the naked eye.
About the presenters

James Anderson is a Global Marketing Manager at Leica Biosystems with experience with histology and scientific, technical, and marketing communications.

Geoffrey Rolls is a Histology Consultant with decades of experience in the field. He is a former Senior Lecturer in histopathology in the Department of Laboratory Medicine, RMIT University in Melbourne, Australia.
Leica Biosystems Knowledge Pathway content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2025 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.