Optimizing Breast Cancer Pathology Report Delivery


Background
Leica Biosystems conducted process assessments to determine if the management of breast tissue across the integrated pathway, from biopsy to diagnosis and report, impacts the ability to render a timely diagnosis.
We found silos within facilities, lack of standardized processes for tissue collection (core needle versus vacuum assisted devices), suboptimal staff scheduling, excessive manual tissue handling and excessive use of inefficient documentation. Opportunities for improvement were identified through benchmarking Academic Medical Centers performing 16,000-53,000 surgeries per year.
Methods
Leica Biosystems team of clinical and process experts conducted on site observations of processes, from the time a patient entered the biopsy suite, until the time their pathology report with diagnosis was delivered. Baseline metrics were captured; including over 300 tissue processing steps, 14 Inter/Intra departmental hand-offs and use of 3 different IT systems. Turnaround time for delivery of pathology report ranged from 4.0 – 10.0 days.
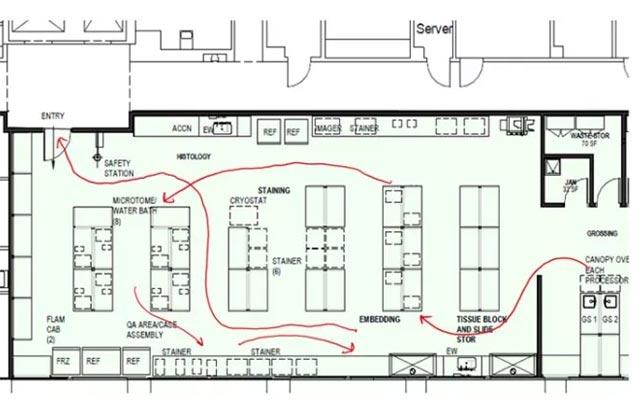
A multidisciplinary team of process and clinical experts met with leadership to implement the following recommendations: utilization of lean processing, aligning staffing schedules, streamlining the physical layout of the facility, automating workflow, implementing a specimen tracking system, and confirming appropriate metrics are monitored.
Results
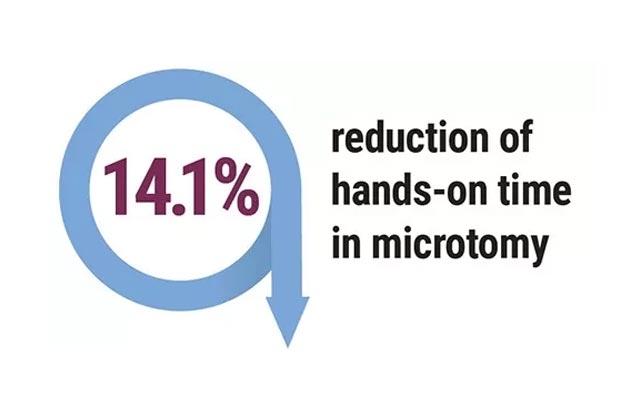
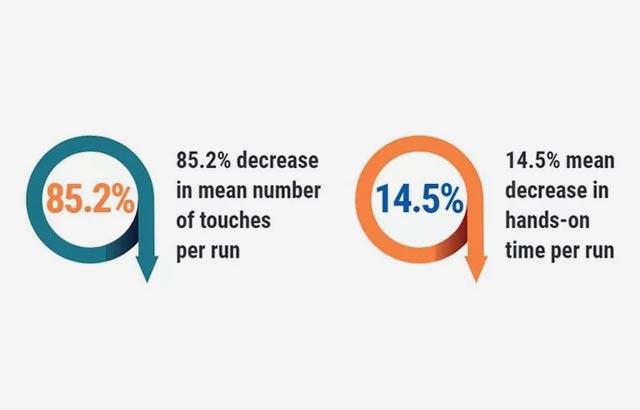
Hospitals were revisited, and the following outcomes were achieved in the labs that followed recommendations; pathology report delivery time decreased to 1.2 - 3.6 days. Additionally, 3,536 hours of lab labor were reallocated, there was a 40% improvement in individual staff productivity, and a 47% reduction of manual specimen handling.
One partner chose not to follow recommendations and upon reassessment a year later; there were no significant changes in their processes or turnaround times. In addition, staff were verbally dissatisfied with their work environment and lack of process improvement.
Conclusion
Pathologic evaluation of breast tissue and report delivery time can be improved by assessing current processes, then (as appropriate) applying lean process principles, embracing automation and system integration, and establishing standardized workflows. The greatest reduction in time was seen through optimization of testing for those samples in need of additional testing (eg. IHC, ISH). Observational data in this pilot study demonstrated how process improvements across the integrated process can result in faster turnaround times resulting in rendering more confident diagnosis and expedited care delivery for the breast cancer patient.
Projections and Realized Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.
발표자 소개

Kimberly Byrwa-Neff is a registered nurse with a background in both clinical nursing and in evidence generation in industry. Her clinical expertise lies in cardiology and oncology, specifically breast cancer. As a nurse, black belt in process excellence, daughter of a breast cancer patient, and survivor of breast cancer herself she brings the full patient experience to her work to improve care for breast cancer patients. Kimberly has authored numerous papers and presented improvement in clinical outcomes as a result of working with breast teams across the country.

Adam Walter at Leica Biosystems is a Certified Six Sigma Master Black Belt and Certified Lean Agent. Adam has worked with over 500 facilities, spanning across the globe, focused on optimizing efficiency, improving quality and integrating automation into clinical workflow processes. His work has traversed a majority of the clinical and anatomic laboratory, as well as hospital departments such as pharmacy, chemotherapy preparation and supply chain. He has multiple publications in the space of workflow and automation as well as authoring industry guidelines relating to laboratory design and architecture.
Related Content
라이카 바이오시스템즈 Knowledge Pathway 콘텐츠는 에서 이용할 수 있는 라이카 바이오시스템즈 웹사이트 이용 약관의 적용을 받습니다. 법적고지. 라이카 바이오시스템즈 웨비나, 교육 프레젠테이션 및 관련 자료는 특별 주제 관련 일반 정보를 제공하지만 의료, 규정 또는 법률 상담으로 제공되지 않으며 해석되어서는 안 됩니다. 관점과 의견은 발표자/저자의 개인 관점과 의견이며 라이카 바이오시스템즈, 그 직원 또는 대행사의 관점이나 의견을 나타내거나 반영하지 않습니다. 제3자 자원 또는 콘텐츠에 대한 액세스를 제공하는 콘텐츠에 포함된 모든 링크는 오직 편의를 위해 제공됩니다.
모든 제품 사용에 다양한 제품 및 장치의 제품 정보 가이드, 부속 문서 및 작동 설명서를 참조해야 합니다.
Copyright © 2025 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.