
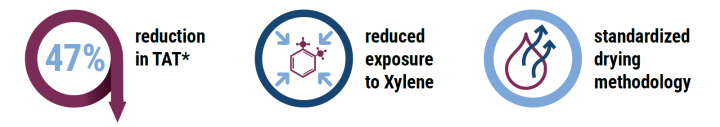
HistoCore Spectra CV coverslipper reduces turnaround time by 47% from glass coverslipping to slide drying completion
During the demonstration using the HistoCore SPECTRA CV Coverslipper, the customer experienced:
Preface
In the current histopathology process, growing workloads are driving the need for standardization, increased productivity and to further reduce technologist hands-on time wherever automation is possible. Thus, this testimonial focuses on evaluating the entire coverslipping workflow, demonstrating that the use of the HistoCore SPECTRA CV Coverslipper built-in oven with active heating technology meets those needs.
Introduction
It is sometimes assumed that film coverslippers enable a faster slide processing time compared to glass coverslippers.
Slide processing time is commonly defined as the time needed for coverslipping only. However, in a real laboratory workflow, the slides need to be completely dry before any further handling.
Furthermore, dried slides are necessary to reduce exposure to hazardous fumes and are an absolute must when using digital pathology.
To meet the requirement of having completely dried slides there are three commonly practiced options:
- Use a coverslipper with a built-in oven
- Remove the slides from a coverslipper to dry in a fume hood (no heat applied) or external oven
- Leave the slides to dry in a coverslipper drying station (no heat applied)
Method
The purpose of this assessment was to evaluate and understand processing times of glass vs. tape coverslipped microscopic slides for diagnostic evaluation. The HistoCore SPECTRA CV Coverslipper (for glass coverslipping) and the Sakura Tissue Tek® Film Coverslipper were used for comparison. This trial was conducted at an independent university pathology laboratory.
The coverslipping and drying process was evaluated for a full load of 60 slides per coverslipper. 60 slides were selected for this trial since it is the maximum number of slides possible to include in one batch for both coverslippers. The process began with loading 60 slides onto each system and ended by the time the slides were completely dry.
Slide dryness was defined by the following factors:
- No xylene residue visible on the slides
- No xylene residue visible between the slides and the staining rack
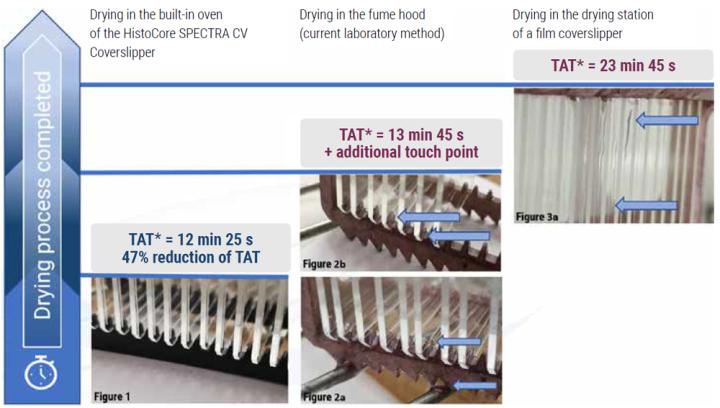
Results
Conclusion
In this assessment we have demonstrated the use of the HistoCore SPECTRA CV Coverslipper built-in oven with active heating technology to reduce TAT* by 47% compared to using the drying station of the existing film coverslipper. Additionally, the laboratory staff reported minimized exposure to xylene fumes due to the reduction of workflow steps and the efficient standardized drying methodology.
Furthermore, results showed a decrease in TAT* by 9.7% versus the commonly practiced workaround of removing the slides from the film coverslipper and placing them under an external fume hood for drying. Thereby the lab was also able to eliminate the additional step in the workflow of slide transportation from the film coverslipper to the fume hood.
*TAT= turnaround time from coverslipping until slide drying complete
Projections and Realized Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.
Want to see how the HistoCore Spectra CV coverslipper can help improve your lab's turnaround time?
El contenido de Leica Biosystems Knowledge Pathway está sujeto a las condiciones de uso del sitio web de Leica Biosystems, disponibles en: Aviso legal.. El contenido, incluidos los webinars o seminarios web, los recursos de formación y los materiales relacionados, está destinado a proporcionar información general sobre temas concretos de interés para los profesionales de la salud y no está destinado a ser, ni debe interpretarse como asesoramiento médico, normativo o jurídico. Los puntos de vista y opiniones expresados en cualquier contenido de terceros reflejan los puntos de vista y opiniones personales de los ponentes/autores y no representan ni reflejan necesariamente los puntos de vista ni opiniones de Leica Biosystems, sus empleados o sus agentes. Cualquier enlace incluido en el contenido que proporcione acceso a recursos o contenido de terceros se proporciona únicamente por comodidad.
Para el uso de cualquier producto, debe consultarse la documentación correspondiente del producto, incluidas las guías de información, los prospectos y los manuales de funcionamiento.
Copyright © 2025 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.