Special Stains – Which One, Why and How? Part I: Mucins and Glycogen

Define “Special Stain”
“Special stain” is a term used to refer to many alternative staining techniques that are used when the traditional H&E does not provide all the information the pathologist or researcher needs from a tissue slide. “Special stains” are processes that generally employ a dye or chemical that has an affinity for the particular tissue component that is to be demonstrated. They allow the presence/or absence of certain cell types, structures and/or microorganisms to be viewed microscopically. “Special stains” are not the same as and existed long before immunohistochemical (IHC) and/or molecular techniques (probes).
Mucins – An Introduction
Mucins are part of a complex group called carbohydrates. They can be simply defined as sugars and starches. Carbohydrates are divided into monosaccharides, polysaccharides and mucopolysaccharides. Mucins are mucopolysaccharides which are explained as long chains of sugar molecules found throughout the body and essential for life and significant in maintaining the structural integrity of bone, cartilage, skin, elastic tissue and membranes They are important in cell growth as they help regulate the flow of nutrients between capillaries and cells and are known as “The Glue of Life”.
There are two broad categories of mucins:
Acid mucins which carry a negative charge on the mucin molecules and can be classified as either simple (carboxyl group added) or complex (sulfuric acid group added). They are found widely throughout the gastrointestinal and respiratory tracts.
Neutral mucins which lack acid groups and carry no charge. They are found in the epithelium of the stomach and the Brunner’s glands of the duodenum. They protect the lining of the duodenum from stomach acid content, help enable absorption by providing an alkaline environment for digestive enzymes, and lubricate intestinal walls and prostate epithelium.
Acid Mucins – The Three Most Often Used Stains to Demonstrate
1) Alcian Blue
Alcian Blue is a basic dye containing copper, which gives it the blue color. The stain molecules carry a positive charge and are attracted to the negative mucins. Adjusting the PH of the Alcian Blue solution allows the demonstration of subtypes of acid mucins.
Ph 2.5 – considered a comprehensive mucin stain will demonstrate:
- Carboxylated (low acidity) simple mucins such as connective tissue and cartilage
- Goblet cells in Barrett’s esophagus
Ph 1.0 will demonstrate sulphated (high acidity) complex mucins in:
- Large intestinal goblet cells
- Bronchial serous glands
- Adenocarcinomas
Examples of tissue normally staining positive for mucin with the Alcian Blue process:
- Intestinal goblet cells
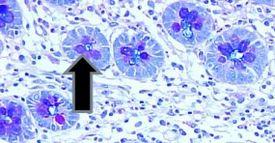
- Barrett’s esophagus goblet cells
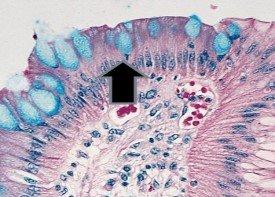
- Mucoid capsules of organisms – Cryptococcus

- Mast cell granules – immunocompetent cells that are found in almost all tissue and function as sentinels of immune responses

Examples of disease processes that are identified by Alcian Blue:
- Mucinous tumors
- Carcinomas that are comprised of at least 60% mucus are referred to as mucinous
- Mucinous tumors comprise about 10 – 15% of all adenocarcinomas
- Adenocarcinoma confirmed as mucin producing
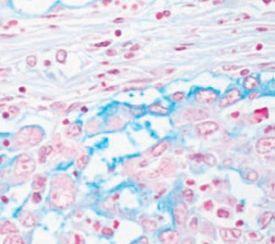
- Myxedema – a type of swelling (edema) with abnormal deposits of mucin in the skin and other tissues
- Myxoma – a rare benign tumor of the heart which can grow to obstruct blood flow and can be fatal
Alcian Blue staining summary:
|
Tissue type |
Composition |
Alcian blue 2.5 |
Alcian blue 1.0 |
|---|---|---|---|
|
GI tract |
Neutral mucins |
Negative |
Negative |
|
Prostate |
Neutral mucins |
Negative |
Negative |
|
Goblet cells |
Acid mucins - Simple |
Positive |
Negative |
|
Tissue Stroma |
Acid mucins - Simple |
Positive |
Negative |
|
Adenocarcinomas |
Acid mucins - Complex |
Negative |
Positive |
|
Cartilage, Bone |
Acid mucins - Complex |
Negative |
Positive PH 0.5 |
Note: Alcian Blue does not stain neutral mucins
The Process

The Protocol – Incubation times may vary per manufacturer
|
1. |
Run slide to water |
|
|
2. |
Alcian Blue – careful to select appropriate PH |
30 min |
|
3. |
Rinse well in running water |
2 min |
|
4. |
Counter stain in nuclear fast red |
2 min |
|
5. |
Rinse briefly in running water |
|
|
6. |
Dehydrate, clear, and mount |
Troubleshooting the Alcian Blue stain:
Nuclear fast red should be mixed well before use for best results as it tends to settle out of solution and will not provide a good counterstain. Make sure you use the appropriate PH of Alcian Blue to prevent a potential false negative.
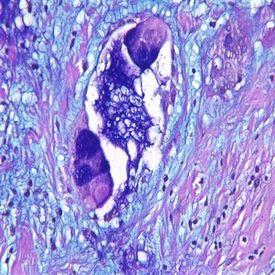
2) Colloidal Iron
Colloidal Iron is used to distinguish acid mucins. It is often used to replace the Alcian Blue stain due to its greater sensitivity for acid mucins with detection of very small quantities. It is used for diagnosing some renal cell carcinomas and some mesotheliomas – a rare form of cancer that develops from transformed cells originating in the mesothelium (protective lining that covers many internal organs).
The Process
Positively charged iron ions (ferric) are attracted to the negative mucin molecules. The attached iron ions are then treated with potassium ferrocyanide/HCL to form visible bright blue deposits.


The Protocol – Incubation times may vary per manufacturer
|
1. |
Run slides to water |
|
|
2. |
Incubate in 12% GLAA (glacial acetic acid) |
12 min |
|
3. |
Straight to working colloidal iron solution |
60 min |
|
4. |
Rinse in 12% GLAA |
3 min |
|
5. |
Rinse in second 12% GLAA |
3 min |
|
6. |
Ferrocyanide – Hydrochloric acid solution |
20 min |
|
7. |
Rinse in running water |
2 min |
|
8. |
Counterstain in nuclear fast red |
5 min |
|
9. |
Rinse briefly in running water |
|
|
10. |
Dehydrate, clear, and mount |
Troubleshooting the Colloidal Iron stain – Dealing with non-specific Hemosiderin staining:
- Prepare two identical slides labeled A and B
- Incubate slide A through the entire protocol
- Incubate slide B once rehydrated in Potassium Ferrocyanide – HCL solution only
- In your analysis of the test tissue, disregard any staining on slide B that may be seen on slide A
3) Mucicarmine – A very specific stain for mucin of epithelial origin
Long before immunohistochemical procedures were available, the Mucicarmine stain was used to verify the presence of signet-ring carcinoma, a rare form of highly malignant adenocarcinoma that produces mucin. It is an epithelial malignancy characterized by the histologic appearance of signet ring cells.
Signet-ring cell carcinoma demonstrated by three methods:

The Mucicarmine stain is also used to differentiate between mucin negative squamous cell carcinoma and mucin positive adenocarcinoma.

Additional example of positive staining with mucicarmine:

The Process
Carmine, a dye obtained from the pulverized bodies of the cochineal beetle, is bound into solution with aluminum salts (a process called mordanting) much the same as hematoxylin. This produces a positively charged complex which is attracted to the negative acid mucin molecule.

The Protocol – Incubation times may vary per manufacturer
|
1 |
Run slides to water |
|
|
2 |
Working Wiegert’s hematoxylin |
5-10 min |
|
3 |
Rinse in running water |
|
|
4 |
Working mucicarmine solution |
20-30 min |
|
5 |
Rinse in running water |
|
|
6 |
Metanil yellow |
1-3 min |
|
7 |
Rinse briefly in running water |
|
|
8 |
Dehydrate, clear and mount |
Troubleshooting the Mucicarmine stain:
Follow manufacturer’s instructions for making working Mucicarmine. Remember to dilute according to instructions and use appropriate water for dilution (distilled vs tap). Leaving it longer in a bad solution or staining in undiluted mucicarmine will not give you the results you want. Use fresh Wiegert’s – if your Wiegert’s is not purple but more brownish, it has broken down and your nuclei will not stain properly. Working Wiegert’s might last a full day but normally not overnight. Go light on the metanil yellow, as it is a counterstain.

Neutral Mucins – The Most Common Stain Used to Demonstrate
Periodic acid Schiff - PAS
Periodic acid - Schiff is the most versatile of the carbohydrate stains demonstrating neutral mucins and as noted below, glycogen. It recognizes non-sulfated, simple acid mucins in epithelial cells and does not rely on the negative charge of the mucin cells but the structure of the saccharide units.
The Process
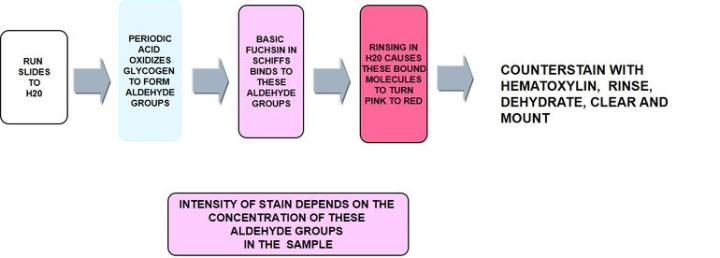
The Protocol – Incubation times will vary per manufacturer
|
1 |
Run slides to water |
|
|
2 |
Oxidize in 0.5% periodic acid |
5 min |
|
3 |
Schiff’s solution |
15 min |
|
4 |
Rinse in running water |
5 min |
|
5 |
Mayer’s hematoxylin |
1 min |
|
6 |
Rinse in running water |
1 min |
|
7 |
Dehydrate, clear, and mount |
Acid Mucins and Neutral Mucins – How to Demonstrate Both on the Same Slide
Alcian Blue – PAS
The Process
Alcian Blue will stain the acid mucins and the neutral mucins will stain PAS positive

Results:
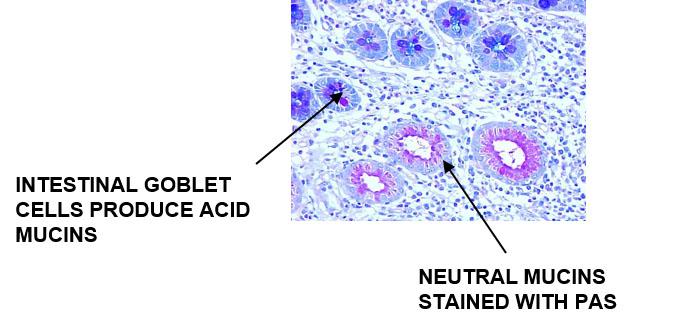
The Protocol – Incubation times may vary per manufacturer
|
1. |
Run slides underwater |
|
|
2. |
Alcian blue |
15 min |
|
3. |
Rinse in running water |
12 min |
|
4. |
Periodic acid |
5 min |
|
5. |
Rinse in running water |
|
|
6. |
Schiff's solution |
10 min |
|
7. |
Rinse in running water |
5 min |
|
8. |
Mayers hematoxylin |
1 min |
|
9. |
Rinse in running water |
|
|
10. |
Dehydrate, clear, and mount |
Troubleshooting the Alcian Blue – PAS:
- Use Alcian Blue at PH 2.5
- Make sure periodic acid is fresh
- Store Schiff’s according to manufacturer’s guidelines
Glycogen – Introduction
Glucose is the main source of fuel for cells. Excess glucose is stored in the liver and muscles. This stored form of glucose is called glycogen. When the body is not getting enough glucose from food, it will break down the glycogen to release glucose into the bloodstream.
How to demonstrate glycogen:
Periodic acid Schiff's – PAS are used to demonstrate glycogen in the liver, the basement membrane, certain tumors of the bladder, kidney, pancreas and ovary, and fungus.
Examples of tissue that normally stain positive for PAS:


The presence of glycogen and its distribution patterns are significant in diseases such as:
-
Glycogen storage disease of the liver
-
Pompe disease - the build-up of glycogen causes progressive muscle weakness (myopathy) throughout the body and affects various body tissues, particularly in the heart, skeletal muscles, liver and nervous system
-
Rhabdomyosarcoma – a connective tissue cancer
-
Mesothelioma
The Process

Troubleshooting PAS:
It is important for periodic acid to remain undiluted and strong. Read Schiff’s package inserts for proper storage
How to demonstrate true glycogen:
PASD – Periodic acid Schiff with diastase
Diastase - a malt extract containing alpha and beta amylase extracts which is an enzyme that aids in the conversion of starches and sugars. This amylase breaks down glycogen into smaller sugar molecules which are then washed out.
How to run a diastase digestion to differentiate glycogen from other PAS positive molecules:
- Place two identical tissue sections - one per slide
- Treat slide A with water or buffer solution
- Treat slide B with diastase (a-amylase 0.25g dissolved in 50 ml distilled water)
- Stain both slides with PAS per protocol above
- Dehydrate, clear, and mount
Results:
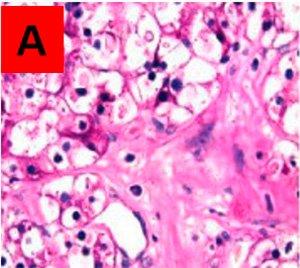

Areas that are PAS positive in the untreated slide A, and PAS negative in the treated slide B, are assumed to be true glycogen. PAS positive in both is other PAS positive material not broken down by the enzyme.
Troubleshooting the PASD:
Make sure the periodic acid is fresh and that the Schiff’s solution is stable. Ensure that the enzyme you are using is correct (it does matter). Dilute the enzyme according to protocol specifications and remember, the PAS positive that disappears after digestion is glycogen. In this case, a negative means a positive.
Tip: If you do not have the proper enzyme commercially available, you can use your own saliva as human saliva is a great digestive enzyme for breaking down glycogen.
About the presenter

Carolyn Doan received her ASCP registration in 1969 after completing classes at Georgia State University and St Joseph school of Histotechnology in Atlanta, Georgia. For 40 years she shared her passion for histology in research (at Yerkes Primate Research Center), in the clinical world (managing the Pathology Department at Florida Hospital in Orlando and serving on the board of the Florida State Licensure Task Force and as president of the Florida State Society for Histotechnology), and in industry (as a Sales executive, Marketing Manager and North American staining sales specialist). Since her retirement in 2013, she founded Creative Histology Consulting.
References
Rolls, Geoffrey. “101 Steps to Better Histology – A Practical Guide to Good Histology Practice”. Knowledge Pathway, Training Resources.
Dorst, Dick. “The Role of Mucopolysaccharides in Good Health.” Aquaculture, INC., 2012.
Shaikh, Imran. "Special Stains in Histopathology” Kem Hospital, 2012.
Ellis, Roy. “Alcian Blue and PAS Staining Protocols.” IMVS Division of Pathology, The Queen Elizabeth Hospital, Woodville, South Australia.
Mowry, RW. J. Histochem. Cytochem., 4, 407, 1956.
Myers, Russell. “Special Stain Techniques for The Evaluation of Mucins.” Knowledge Pathway, Tutorials.
Pernick, Nat . “Mucins.” Pathology Outlines Jan 26, 2016.
Sheehan, D. . “Theory and Practice of Histotechnology.” Carbohydrates. 2nd edition, 9: 159-178.
Related Content
Leica Biosystems Knowledge Pathway content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2025 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.