Modern Multiplex Solutions for the Research Lab
Multiplexing addresses the need for researchers to assess multiple biomarkers (protein and/or nucleic acid markers) at specific locations within a tissue sample. The information revealed through simultaneous detection of multiple markers, the spatial relationships among cells and tissue in disease, and the heterogeneity are now understood to be critical to developing effective therapeutic strategies.
The latest technology encompasses multiplex IHC as well as multiplex ISH and FISH.
Key considerations for choosing to Multiplex
- The need to extract the maximum amount of data from a limited sample, multiplex technology enables the user to detect many biomarkers in a single tissue section.
- Multiplexing can help determine which targets are important, by starting with a large range of potential markers and using the resulting spatial data to refine to the critical few.
- Multiplex staining on tissue provides cell-specific context that molecular techniques and PCR can’t provide.
- Tissue multiplexing visualizes both protein and nucleic acid targets in the same tissue section.

The Fundamentals
The Difference Between Number of Colors, Plex, and Multiplexing
Number of colors: Every single stain on the slide including counterstains
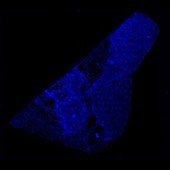
DAPI (counterstain)
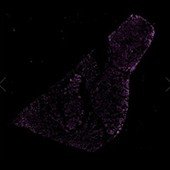
CY7
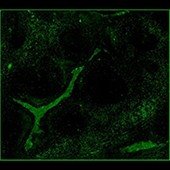
Spectrum Green
Spectrum Orange
Plex: The number of targets to be analyzed excluding counterstain
Dual or 2-plex red and brown IHC with crystal light green counterstain
Dual or 2-plex red and green RNA ISH with hematoxylin counterstain
Multiplexing: the ability to simultaneously detect 2 or more markers on a single slide (eg CD3, CD4, CD8 & counterstain). The nuclear counterstains most frequently used are hematoxylin for brightfield and DAPI for fluorescent microscopy
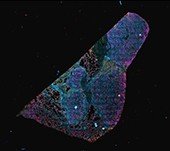
Ultivue PD-L1 kit staining human lung, 4-plex plus DAPI (The protocol was carried out on the BOND RX fully automated stainer from Leica Biosystems and the stained tissue imaged on the Aperio VERSA whole slide scanning system)
Fluorescence Multiplexing
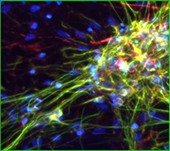
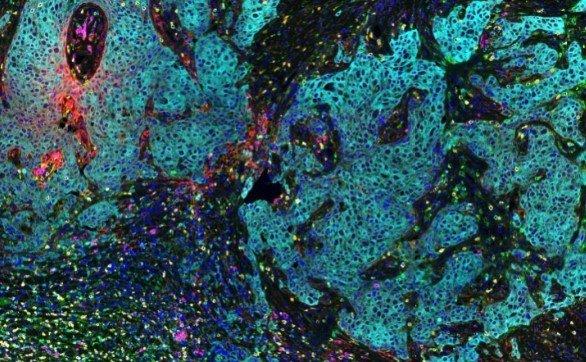
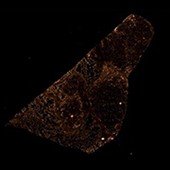
Rat Neuron, 6-plex
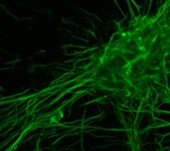
Spectrum Green
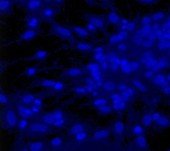
DAPI
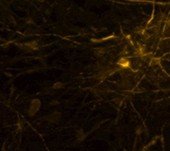
Spectrum Orange
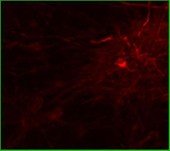
Texas Red

CY7
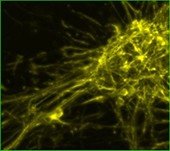
CY5
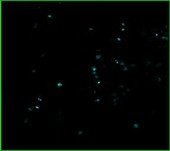
Aqua
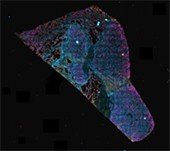
Ultivue PD-L1 kit staining human lung, 4-plex plus DAPI (The protocol was carried out on the BOND RX fully automated research stainer and the stained tissue imaged on the Aperio VERSA whole slide scanning system)
Chromogenic Multiplexing
Chromogenic Multiplexing (multiplex IHC and ISH)
Chromogenic multiplexing provides the ability to look at 3 or more markers on the same slide using brightfield microscopy. Chromogens provide a more stable and permanent result compared to their fluorescent counterparts.
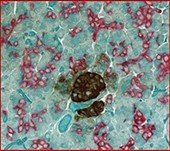
Normal human tonsil stained with:
Marker 1 - CyclinD1 - Red
Marker 2 - CD20 - Brown
Counterstain = Hematoxylin
Normal human tonsil stained with:
Cyclin D1 red nuclear
Normal human tonsil stained with:
CD20 brown membranous stain
Normal human tonsil stained with:
Hematoxylin counterstain

Normal tonsil tissue stained with:
Marker 1 - PD-L1 - Red
Marker 2 - CD68 - DAB
Marker 3 - CD8 - Blue
Marker 4 - Pan-CK - Green
Counterstain = Hematoxylin

Melanoma in skin stained with:
Marker 1 - PD-L1 - Red
Marker 2 - CD68 - DAB
Marker 3 - CD8 - Blue
Marker 4 - Pan-CK - Green

Melanoma in skin stained with:
Marker 1 - PD-L1 - Red
Marker 2 - CD68 - DAB
Marker 3 - CD8 - Blue
Marker 4 - Pan-CK - Green

Human bladder stained with:
Marker 1 - CK20 - Red
Marker 2 - p53 - Brown
Marker 3 - CD44 - Green
Counterstain = Hematoxylin

Adenocarcinoma in human colon stained with:
Marker 1 - CDX2 - Red
Marker 2 - CD3 - Green
Counterstain = Hematoxylin
Multiple Use Cases

Immuno-Oncology
Get the full picture for your tumor microenvironment research
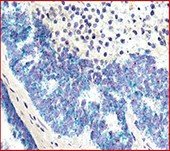
Immuno-oncology has been one of the primary drivers of the current multiplex technology development. Multiplex immunohistochemical (IHC) analysis of formalin-fixed, paraffin-embedded (FFPE) tissue samples enables researchers to study the spatial relationships between different cell phenotypes in situ. Tonsil is often the first step to check that the antibodies are identifying the appropriate immune cells. After that, the actual tumor microenvironment can be visualized with hematologic samples, staining often determines cell lineage/origin as well. This phased process of optimization is long but ensures fidelity of results.
Fluorescence In Situ Hybridization (FISH) Assay
Nucleic Acids in Cancer research
FISH is a sensitive, accurate, and reliable technique widely applied in cancer research. The genetic defects uncovered by FISH represent early genetic triggers or events responsible for cancer at stem-cell level. FISH provides cell-based context for specific genomic aberrations and plays an important role in detecting specific biomarkers in solid and hematologic neoplasms
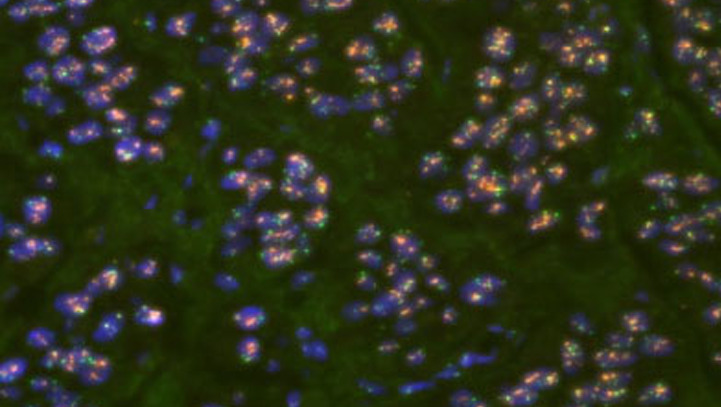

Quantitative Multicolor QM- FISH
Pairs of probes have been conventionally used to detect a single genetic event like deletion or amplification of a locus or chromosomal translocation. However, with the discovery of multigenic diseases including cancer, simultaneous detection of such genes by using multiple probes on a single slide aids understanding of disease progression (quantitative multicolor FISH).
Tissue Heterogeneity
It is easy to think of heterogeneity between tumor and non-tumor tissue, but it is also well known that there is heterogeneity within the tumor itself. Not every cell will stain the same and not every marker will be present in the same cells. Multiplex IHC and ISH uncover this multi-faceted approach to tissue heterogeneity and provide context.
Example of breast cancer image using Aperio Image Analysis software.

Educational Resources
Tips & Tricks to Multiplexing: How to Choose Chromogen Colors for Multiplex and Detection Systems for Multiplex Assays
Tips & Tricks to Multiplexing: Top 5 Reasons to Multiplex and Chromogenic versus Immunofluorescent Detection
Selecting the right detection for your IHC/ISH project (Fluorescence vs Chromogenic Staining)
Featured Products
BOND RXm
Helping academic researchers achieve more: BOND RXm – Fully Automated Advanced Staining
Aperio VERSA
Delivering Excellence in IHC, ISH and Fluorescent Tissue-Based Research including Multiplex Whole Slide Scanning
For Research Use Only. Not for use in diagnostic procedures.
The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2025 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.






