Optimizing Breast Cancer Pathology Report Delivery


Background
Leica Biosystems conducted process assessments to determine if the management of breast tissue across the integrated pathway, from biopsy to diagnosis and report, impacts the ability to render a timely diagnosis.
We found silos within facilities, lack of standardized processes for tissue collection (core needle versus vacuum assisted devices), suboptimal staff scheduling, excessive manual tissue handling and excessive use of inefficient documentation. Opportunities for improvement were identified through benchmarking Academic Medical Centers performing 16,000-53,000 surgeries per year.
Methods
Leica Biosystems team of clinical and process experts conducted on site observations of processes, from the time a patient entered the biopsy suite, until the time their pathology report with diagnosis was delivered. Baseline metrics were captured; including over 300 tissue processing steps, 14 Inter/Intra departmental hand-offs and use of 3 different IT systems. Turnaround time for delivery of pathology report ranged from 4.0 – 10.0 days.
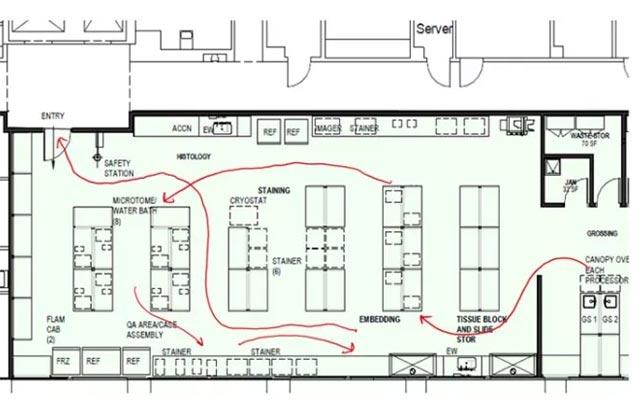
A multidisciplinary team of process and clinical experts met with leadership to implement the following recommendations: utilization of lean processing, aligning staffing schedules, streamlining the physical layout of the facility, automating workflow, implementing a specimen tracking system, and confirming appropriate metrics are monitored.
Results
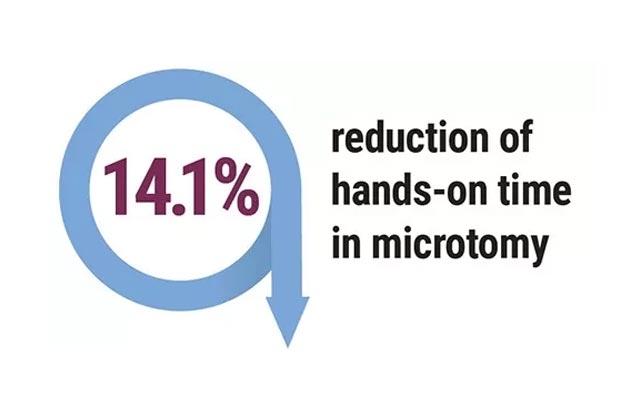
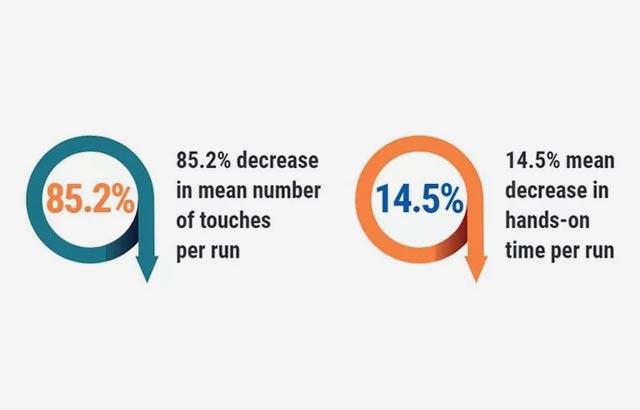
Hospitals were revisited, and the following outcomes were achieved in the labs that followed recommendations; pathology report delivery time decreased to 1.2 - 3.6 days. Additionally, 3,536 hours of lab labor were reallocated, there was a 40% improvement in individual staff productivity, and a 47% reduction of manual specimen handling.
One partner chose not to follow recommendations and upon reassessment a year later; there were no significant changes in their processes or turnaround times. In addition, staff were verbally dissatisfied with their work environment and lack of process improvement.
Conclusion
Pathologic evaluation of breast tissue and report delivery time can be improved by assessing current processes, then (as appropriate) applying lean process principles, embracing automation and system integration, and establishing standardized workflows. The greatest reduction in time was seen through optimization of testing for those samples in need of additional testing (eg. IHC, ISH). Observational data in this pilot study demonstrated how process improvements across the integrated process can result in faster turnaround times resulting in rendering more confident diagnosis and expedited care delivery for the breast cancer patient.
Projections and Realized Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.
About the presenters

Kimberly Byrwa-Neff is a registered nurse with a background in both clinical nursing and in evidence generation in industry. Her clinical expertise lies in cardiology and oncology, specifically breast cancer. As a nurse, black belt in process excellence, daughter of a breast cancer patient, and survivor of breast cancer herself she brings the full patient experience to her work to improve care for breast cancer patients. Kimberly has authored numerous papers and presented improvement in clinical outcomes as a result of working with breast teams across the country.

Adam Walter at Leica Biosystems is a Certified Six Sigma Master Black Belt and Certified Lean Agent. Adam has worked with over 500 facilities, spanning across the globe, focused on optimizing efficiency, improving quality and integrating automation into clinical workflow processes. His work has traversed a majority of the clinical and anatomic laboratory, as well as hospital departments such as pharmacy, chemotherapy preparation and supply chain. He has multiple publications in the space of workflow and automation as well as authoring industry guidelines relating to laboratory design and architecture.
Related Content
Die Inhalte des Knowledge Pathway von Leica Biosystems unterliegen den Nutzungsbedingungen der Website von Leica Biosystems, die hier eingesehen werden können: Rechtlicher Hinweis. Der Inhalt, einschließlich der Webinare, Schulungspräsentationen und ähnlicher Materialien, soll allgemeine Informationen zu bestimmten Themen liefern, die für medizinische Fachkräfte von Interesse sind. Er soll explizit nicht der medizinischen, behördlichen oder rechtlichen Beratung dienen und kann diese auch nicht ersetzen. Die Ansichten und Meinungen, die in Inhalten Dritter zum Ausdruck gebracht werden, spiegeln die persönlichen Auffassungen der Sprecher/Autoren wider und decken sich nicht notwendigerweise mit denen von Leica Biosystems, seinen Mitarbeitern oder Vertretern. Jegliche in den Inhalten enthaltene Links, die auf Quellen oder Inhalte Dritter verweisen, werden lediglich aus Gründen Ihrer Annehmlichkeit zur Verfügung gestellt.
Vor dem Gebrauch sollten die Produktinformationen, Beilagen und Bedienungsanleitungen der jeweiligen Medikamente und Geräte konsultiert werden.
Copyright © 2025 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.